- the first monoclonal antibody, rituximab (MAB) was approved for non-Hodgkin lymphoma in 1997,
- Every living cell has a critical enzyme called Protease. The first Protease inhibitor (PI) was approved for myeloma in 2003. It is bortezomib (ZOMIB)
- The first immuno-modulatory drug, lenalidomide ( IMID) was approved for myeloma in 2006. Remember Thalidomide.
- NAB is a Nano particle Albumin Binder. The first Nanoparticle albumin-bound drug, nab-paclitaxel (Nabs) came for breast cancer in 2005
- Imatinib is a Tyrosine Kinase Inhibitor (TKI). So is Alectinib. In 2001, Imatinib was approved for Philadelphia chromosome positive CML.
The list of cancer drugs have exploded lately. In this article we shall dissect the names of some common biologics and related drugs. This helps in the pronunciation and prediction of their properties.
The MABs
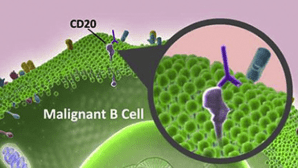
Mabs are Larger complex molecules. They are antibodies ( ie proteins) that selectively bind to malignant cells circulating inside our body and destroy them. Just like vaccines that binds to the spike protein of Covid-19 virus. They must be injected parenterally.
There have been more than 30 MAB molecules approved for cancer treatment today.
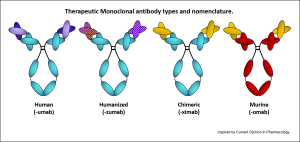
WHO has published a convention guiding how to name a Mab depending on its source. Depending on whether it is of human origin ( or humanized), murine origin or a chimera they become Umab, Zumab, Ximab or Omab. ( See pic)
The MIBs ( Zomibs)
The suffix “zomib” is the designation for protease or proteasome inhibitors. Mibs are small molecules that work inside cancer cells. Each living cell needs protease enzyme for its survival. Zomibs block the protease enzyme of malignant cells to kill them.
Example: Ixazomib, Bortezomib, Carflizomib
NIBs
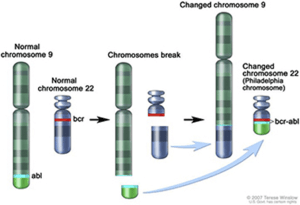
In 2001, Imatinib was approved for Philadelphia chromosome positive CML. In Chronic Myeloid Leukaemia patients the BCR part of chromosome 22 is translocated and fused with ABL of chromosome 9 gene.(pic). It acts on the Tyrosine Kinase enzyme of leukaemia cells.
Today we have a varieties of TKIs all suffixed with inib. Tinibs are shorthand for Tyrosine Kinase Inhibitor another rate limiting enzyme for cancer growth. ( Inib : inhibits)
Alectinib (Alecensa; Genentech) is approved for advanced, non-small Lung cell cancer, Cobimetinib (Cotellic; Genentech) for advanced melanoma, Levatinib (Lenvima; Eisai Inc.) for advanced thyroid cancer, and Osimertinib (Tagrisso; AstraZeneca) for non-small cell lung cancer.
Utility:
Biologic and related drugs are most commonly used in oncology and immune system disorders or diseases. Often, the drug name gives information about the pharmacology and pharmacokinetics. For example, all mabs are proteins, which must be administered parenterally. Nibs are substrates of cytochrome P450 enzymes and therefore liable to drug interactions. Chances of allergic reactions are greater with mabs, especially in drugs that are not fully human. Adverse effects are related to the system targeted (eg, epidermal growth factor receptor antagonists would be likely to cause rash and diarrhea).